About the project
Our vision of the future
Predictive personalized Modeling
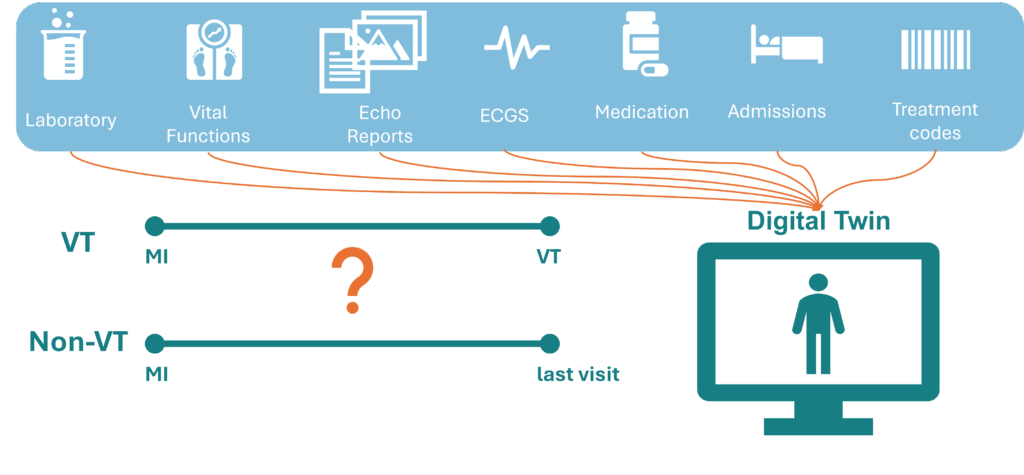
Post-myocardial infarct (post-MI) patients have an increased risk for scar-based ventricular tachycardia (VT), an abnormal heart rhythm, which may lead to sudden cardiac death (SCD). Ablation therapy is used to treat VTs, but has moderate success rates of 50- 80%. Secondly, an implanted cardioverter defibrillator (ICD) can help prevent SCD if ablation is inexecutable. Current guidelines for ICD treatment are based on left ventricular ejection fraction but prove to be insufficient in identifying patients with a high risk of VT. The electro-mechanical interaction of the heart is key to understand VT development. Here, patient-specific computational models can be used to create a cardiac Digital Twin which can serve as a decision support tool in the clinic.
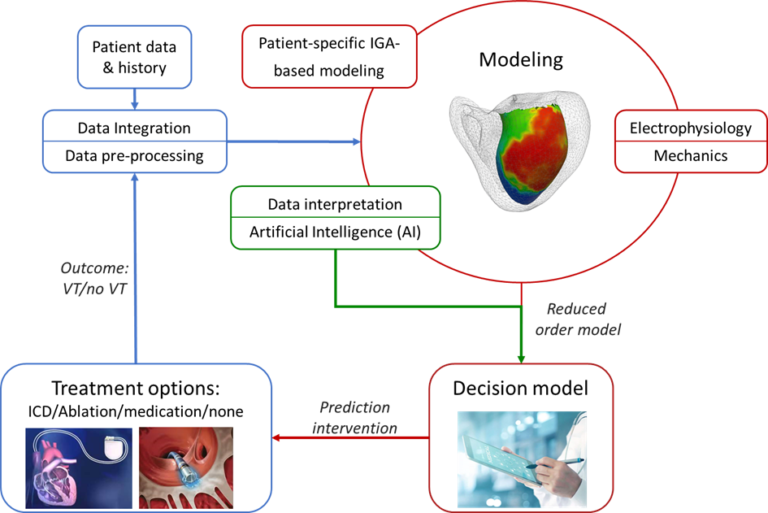
Our project uses Data Integration on patient data & history, for Patient-specific IGA modeling, Electro-mechanically coupled modeling and Artificial Intelligence modeling. The resulting decision model can be used to improve the understanding and prediction of the occurrence of Ventricular Tachycardias and provide optimized patient-specific treatment using computationally efficient models.
Data Integration & Pre-processing
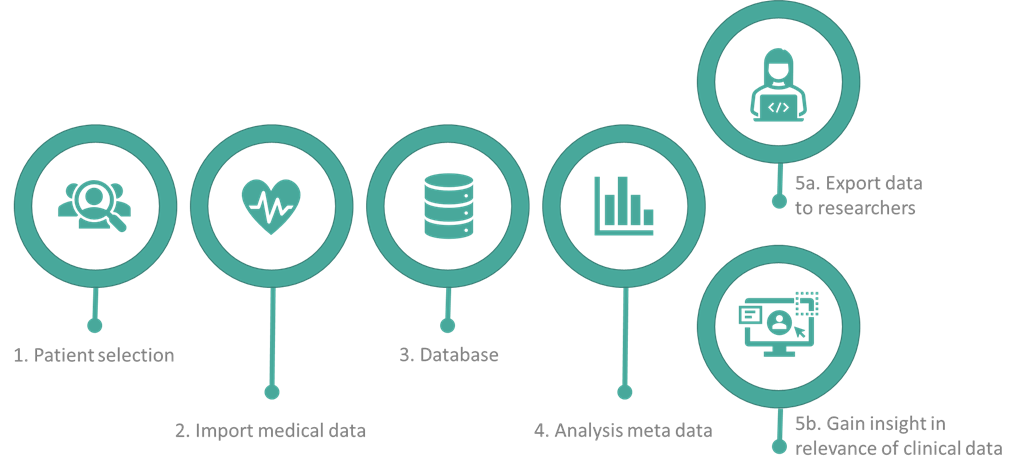
The linking pin between the technological research performed by the PhD students and the clinical application: A clinical workflow is developed in order to acquire patient-specific data. In this workflow, the data is semi-automatically obtained, analyzed and processed to be used for the physiological and data models of the COMBAT-VT project. This workflow has five essential components:
- Selection of patients;
- Obtaining the data from the different information systems at the hospital;
- Saving data according to the FAIR principles;
- Analyzing the data to explore what is exactly saved from the patients and what is useful for the models;
- Preprocessing the data and sharing the data with the PhD researchers working at the TU/e and providing insight with a clinical data interface.
Melissa Niemantsverdriet & Marloes de Winter
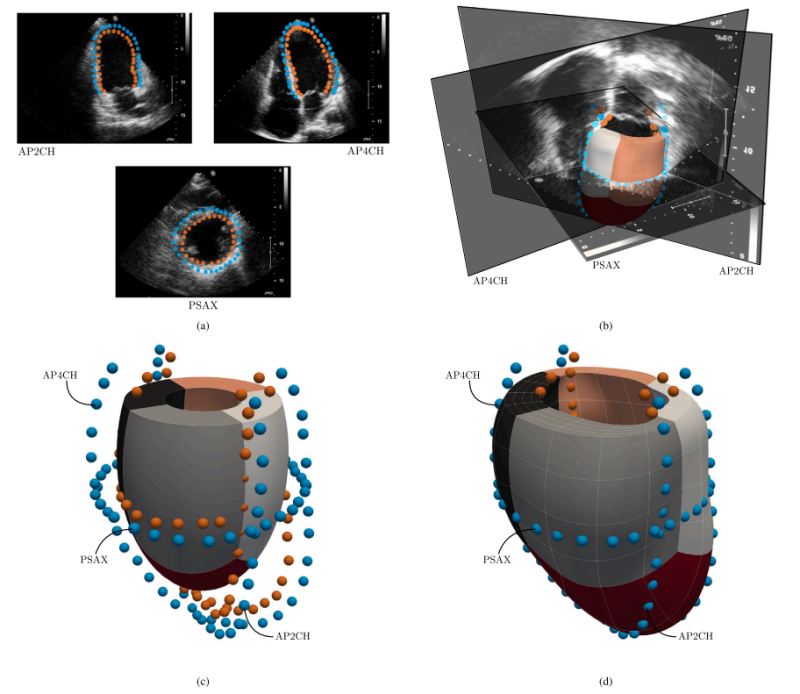
Patient-specific IGA-based modeling
To study potential mechanisms involved in VTs, new methods are required that enable high-fidelity analyses in a computationally
efficient and accurate manner. Additionally, incorporating these methods into the clinical workflow should allow for the rapid output of indicators that support clinical decision-making. We aim to achieve the goals by: (i) developing a computationally robust, efficient, and accurate framework that provides a basis to investigate potential mechanisms of VTs, subject to varying input, particularly regarding geometric data. Use is made of the Isogeometric Analysis (IGA) paradigm, to assess whether it offers significant benefits compared to the traditional finite element (FE) approach. (ii) Developing a reduced-order model that limits computation costs, while providing quantitative indicators of the cardiac behavior accompanied by a measure of uncertainty. In doing so, we leverage the IGA-based full-order model results to train and enrich the parameters of an established semi-analytical model using Bayesian inference. It interpolates new parameter values using Gaussian Processes. Once properly trained, the reduced-order model provides a cardiac response within specified uncertainty
bounds in a fraction of the time required for the full-order simulations.
The developed methods enhance the ability to test new hypotheses in cardiac mechanics, particularly those that require multiple computations or the incorporation of additional physics. This accelerates computational research of various disease treatments, ultimately benefiting clinical research. Furthermore, the developed framework can be extended to more advanced cardiac models and, in principle, is also applicable to other engineering fields.
Robin Willems
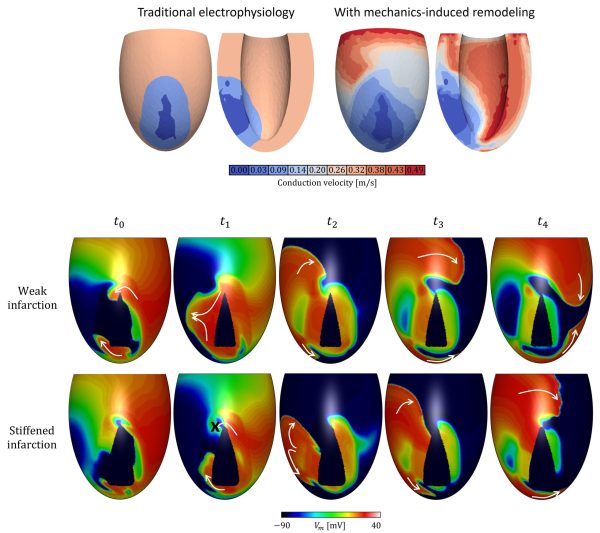
Electro-mechanical modeling
In traditional electrophysiology models, remodeling of electrical properties is taken into account at the infarct area only. In our project, information from mechanical simulations is considered too by implementing conduction slowing in areas with abnormal mechanics, which can extend beyond the structural infarct area. The hypothesis that strain affects connexin density, and therewith conductivity, is central to this project. It results in a more heterogeneous substrate for ventricular tachycardia. We use this hypothesis to test whether in can explain the long time frame between infarction and the onset of VT (sometimes up to several years), by simulating infarct stiffening to obtain strains corresponding to the chronic infarct state. Furthermore, the effects of the hypothesis on ablation therapy success is explored.
Evianne Willems
Data modeling
Currently, physicians primarily focus on the ejection fraction to assess the risk of ventricular tachycardia (VT) in patients. However, this measurement has proven to be suboptimal in accurately identifying high-risk patients. Hospitals have access to a wealth of data, including echocardiograms, MRI scans, ECG readings, and lab values. We hypothesize that there are subtle changes in these measurements over time that physicians may not be aware of, which could be more effective in predicting VT. Data-driven models, such as those powered by Artificial Intelligence (AI), can play a crucial role in uncovering these potential predictors. AI excels at identifying hidden patterns in data without requiring predefined knowledge, which is precisely what is needed in this context. However, AI models are often hindered by noisy and missing data and may struggle with inter- and extrapolation.
In addition to patient data, there is also physiological and physical knowledge available through computer models developed by projects like those of Evianne and Robin. Ideally, we would combine these computer models with data models to create a VT Digital Twin. A Digital Twin is a predictive computer model that dynamically integrates the physical and digital worlds to represent a patient, continuously updating with new data. Importantly, this Digital Twin should be capable of evolving over time.
To achieve this, we will develop a unique retrospective dataset by collecting multimodal data from post-infarction patients over time. Some of these patients developed VT, while others served as a control group. We will investigate whether temporal changes in the data can predict VT using both statistical and AI models. This approach will help us identify which data is valuable for our Digital Twin. Ultimately, we aim to develop a proof of concept for a Digital Twin that evolves over time, integrating both patient data and physiological models to provide a comprehensive and dynamic representation of a patient’s condition.
Carlijn Buck